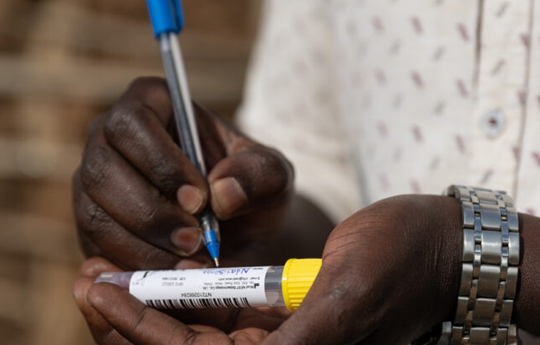
NEST Product is adopted by WHO, Uganda in the clinic trial on three candidate Ebola Vaccines, which is launched by the World Health Organization collaborating with the government of Uganda. As is revealed by WHO, the first doses of the three candidates vaccines against the Ebola Virus Sudan are expected to arrive in Uganda in the following week.

Scientists prepare to take a sample from a 3-year-old boy suspected of dying from Ebola in Mubende, Uganda.
LUKE DRAY/GETTY IMAGES from STATNews
https://www.statnews.com/2022/11/16/who-uganda-plan-to-test-three-candidate-ebola-vaccines-in-outbreak/?utm_campaign=twitter_organic&utm_medium=social&utm_source=twitter&s=09

NEST Viral Transport Medium (VTM)
According to STAT News, the outbreak in Uganda has been cropping up since early August, and later Uganda declared an outbreak ofSudan ebolavirus on 20 September, which has resulted so far in at least 163 confirmed and probable cases and 77 confirmed and probable deaths. So the start of vaccine trials turns into a critical moment towards the development of an effective tool against the current Ebola outbreak in Uganda.
Ebola Virus Disease (EVD) & Ebola Virus Sudan
The Ebola virus causes an acute, serious illness which is often fatal if untreated. EVD first appeared in 1976 in 2 simultaneous outbreaks, one in what is now Nzara, South Sudan, and the other in Yambuku, DRC. The latter occurred in a village near the Ebola River, from which the disease takes its name.Within the genus Ebolavirus, six species have been identified: Zaire, Bundibugyo, Sudan, Ta? Forest, Reston and Bombali. Unlike the Zaire ebolavirus which has sparked most of the recent outbreaks, there are no approved vaccines or therapeutics for the Sudan ebolavirus.
About NEST
It is a great honor of NEST to serves in the trials of Ebola Vaccines in Uganda, which is a manifestation of NEST global popularity and premium quality. NEST, based in NJ, USA, is a global and cutting-edge lab consumables manufacturer and medical service provider.
NEST Three Product lines:
1) Premium lab consumables 2) High-end medical devices 3) Pharmaceutical packaging
Top Products
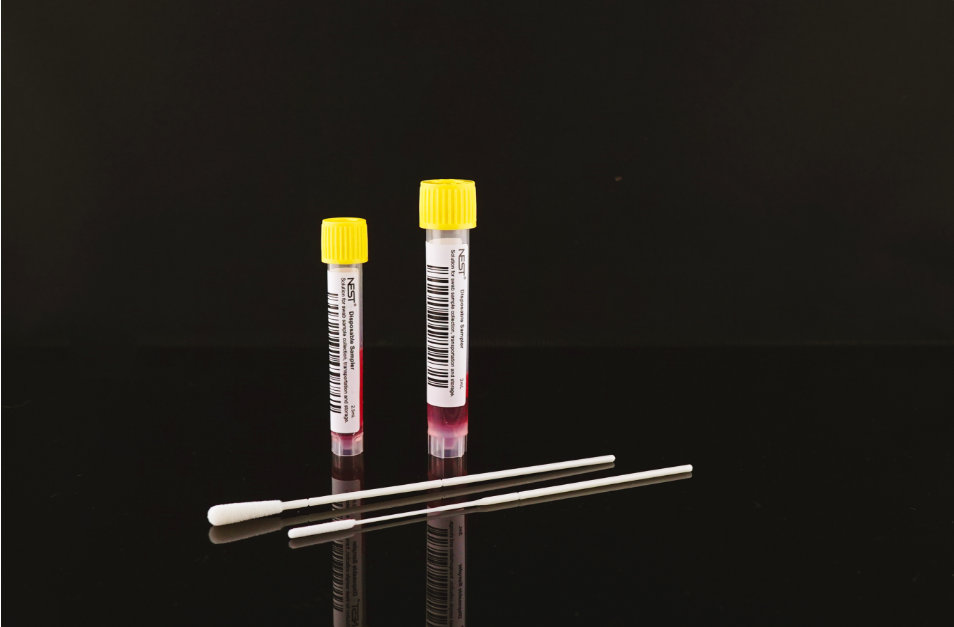
Disposable Sampler
Viral Transport Medium (VTM)
Features
1. Used for collection, storage and transportation of human nasopharyngeal virus samples.
2. Unused VTM medium can be transported at room temperature.
3. Sample transport temperature requirement & storage time: 2 to 4 C°, 48 h.
Inactivation Transport Medium (ITM)

Features
1. ITM medium serves to both inactivate viruses and prevent nucleic acid degradation.
2. Room temperature transport
3. Samples can be stored at room temperature for 20 days.
4. Used for collection, storage and transportation of viruses, chlamydiae, mycoplasma and ureaplasma.
AMIES Transport Medium (ATM)

Features
1. Simplify specimen collection, transport and processing.
2. Fewer samples need to be collected offering a more comfortable experience.
3. Better preservation ability: the colony number of samples was reduced by no more than 1.5log10 after preservation for 48h.
Universal Transport Medium (UTM)

Features
1. Simplify specimen collection, transport and processing.
2. Used for collection, storage and transportation of viruses, bacteria, chlamydia, mycoplasma and urea plasma.
3. The specimen transported in the universal transport media can be used in the laboratory to perform pathogen isolation and detection.
4. Disposable use.
Saline Transport Medium

Features
1. Non-toxic, safe and effective for home collection.
2. Produced according to the FDA standard.
3. Room temperature for transportation and storage.
4. After collection of the sample transportation requirement: room temperature within 48h.
5. Used for collection, storage and transportation of viruses, chlamydia, mycoplasma and urea plasma.
6. Validation: 2 x LoD (low positives) and 10 x LoD (high positives) virus samples for 56 hours ( 40℃ for 12 hours, then 32℃ for 42 hours) in this product can be detected by RT-PCR.
Nasopharyngeal Swab & Oropharyngeal Swab

